Thesis
The field of brain–computer interfaces (BCIs) has evolved from decades of academic research into early-stage clinical and commercial testing. Following Hans Berger’s discovery of brain electrical activity in 1924 and Jacques Vidal’s coining of the term “brain-computer interface” in the 1970s, the field advanced through key milestones, including Phillip Kennedy’s first human implant in 1998 and neural prosthetic control demonstrations in 2012. After two decades of primarily academic development, BCIs are progressing into regulated human trials and early commercialization.
Advances in materials engineering, artificial intelligence, and computing have accelerated this transition. For example, in materials science, scientists developed a wearable microneedle BCI small enough to fit between hair follicles, enabling painless, stable brain-signal detection through the skin without surgery. On the implantable side, Axoft’s Fleuron, an ultra-soft, biocompatible material, reduces tissue scarring and electrode migration while maintaining strong neural contact. These advances exemplify a broader shift toward flexible, nanoscale materials and precision insertion techniques, including robotic and nanorobotic-assisted implantation, that minimize tissue damage and improve long-term stability.
Artificial intelligence advances also have the potential to improve BCI signal interpretation and responsiveness. Between 2022 and 2024, the cost of running models at GPT-3.5 performance levels dropped over 280-fold, while hardware costs declined 30% per year and energy efficiency improved 40% annually. AI performance on benchmarks like MMMU, GPQA, and SWE-bench rose by 18.8, 48.9, and 67.3 percentage points, respectively, reflecting gains in multimodal reasoning. These capabilities are being applied to BCIs: In September 2025, UCLA engineers developed a wearable, noninvasive system that pairs EEG decoding with a camera-based AI model to infer user intent, allowing robotic arms and cursors to move more efficiently. Broader algorithmic progress in multimodal transformers and sequential modeling further enhances BCIs’ ability to decode brain activity across visual, speech, and motor domains.
The global BCI market was valued at $2 billion in 2023 and was projected to reach $6.2 billion by 2030, growing at a 17.8% CAGR. Demand has been driven by neurological and neurodegenerative conditions such as dementia, Parkinson’s disease, epilepsy, and spinal cord injury. The World Health Organization estimates 50 million people lived with dementia in 2017, expected to rise to 152 million by 2050, while about 15 million lived with spinal cord injuries as of 2024, and 1.7% of the US population experienced paralysis as of 2016. These conditions severely limit communication and mobility, underscoring medical demand for BCIs that restore lost function.
Neuralink is developing a fully integrated, implantable BCI to restore neurological function and, eventually, enhance human capability. Its system combines neural recording, robotic implantation, and real-time signal processing for applications in paralysis, with future extensions to vision and speech restoration. Founded in 2016 by Elon Musk, the company has begun human trials and raised $650 million in Series C funding to expand clinical testing and manufacturing.
Founding Story
Neuralink was founded in 2016 by Elon Musk with a team of eight scientists and engineers, assembled after Musk met with over 1K potential candidates. The founding group brought expertise across neuroscience, microscale electronics, and biomedical engineering, and included Dongjin (DJ) Seo (President and CEO), Max Hodak, Benjamin Rapoport, Paul Merolla, Philip Sabes, Tim Gardner, Tim Hanson, and Vanessa Tolosa. Together, they set out to build a brain–computer interface (BCI) capable of connecting the human brain directly to digital systems.
Musk’s interest in neural interfaces originated from his growing concern over the rapid advancement of artificial intelligence. In 2014, he warned that AI could become “more dangerous than nukes,” arguing that humans risked being outpaced by machine intelligence. Inspired by the concept of neural lace from Iain M. Banks’ The Culture series, Musk envisioned a high-bandwidth interface that could allow direct communication between humans and computers, enabling what he described as “symbiosis with artificial intelligence.”
To realize this vision, Musk recruited Seo, whose doctoral research at UC Berkeley had produced Neural Dust, a wireless, ultrasonic sensor small enough to operate inside the body. His work demonstrated early feasibility for distributed, low-power implants and earned him recognition on MIT Technology Review’s Innovators Under 35 list. Musk also brought on Hodak, a neural engineering enthusiast who had studied at Duke University and previously founded Transcriptic (later Strateos), a cloud robotics platform for life sciences. Hodak’s combination of technical and entrepreneurial experience led him to serve as Neuralink’s first president.
Neuralink officially launched in April 2017 with the mission of developing advanced brain devices to treat neurological disorders, while maintaining a long-term goal of enhancing human cognition. Neuralink acquired the “Neuralink” trademark from neuroscientists Pedram Mohseni and Randolph Nudo earlier that year and began operating from San Francisco’s Mission District before relocating to Fremont, California.
By 2021, most of the original founding team had departed, citing the company’s demanding pace and divergent priorities. Hodak left to start Science, another neurotechnology venture, leaving Seo as the remaining co-founder alongside Musk. Merolla left in 2022 to later found MK1, an enterprise AI company. Since then, Neuralink has expanded its operations, shifting incorporation to Nevada and beginning construction of new facilities near Austin, Texas, as of July 2024. Its short-term objective is to restore function for patients with severe neurological injuries and diseases. The long-term vision remains the creation of a generalized brain platform capable of bridging human cognition and artificial intelligence.
Product
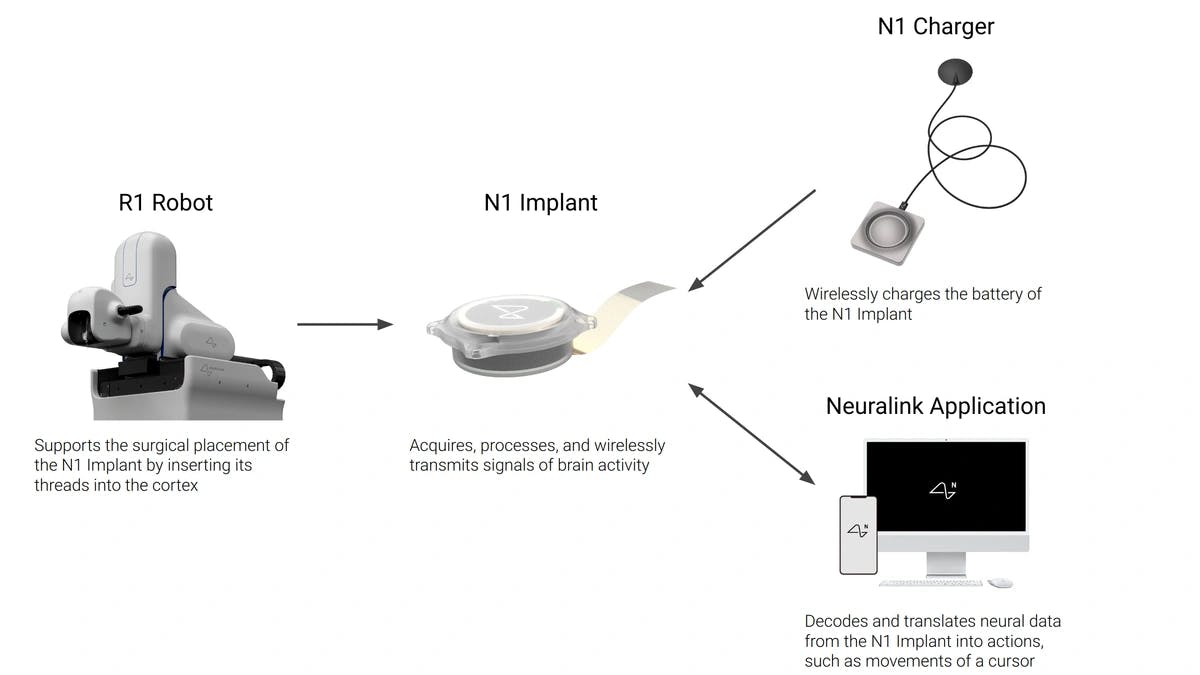
Source: Neuralink
Neuralink is developing a fully integrated brain–computer interface designed to restore lost neurological function and, in the long term, augment human capability. Its first application focuses on restoring function in people with paralysis through the Link system, which records and interprets neural activity from the motor cortex to enable digital control through thought. An implant is surgically placed using a high-precision robotic system that inserts flexible threads into the brain while avoiding blood vessels. Neural signals are wirelessly transmitted to an external application, where they are processed and translated into digital outputs such as cursor movement.
The same platform architecture can be extended to other regions of the brain for applications such as vision and speech restoration. As the technology matures and demonstrates consistent safety in clinical settings, Neuralink plans to explore voluntary implantation for cognitive or physical enhancement.
Product Suite
As of November 2025, Neuralink’s development centered on Telepathy, its first-generation brain–computer interface designed to record and decode movement-related signals from the motor cortex. The implant has been tested in people with paralysis since January 2024 through an early feasibility study that aims to refine the hardware, surgical robot, and decoding software before advancing to a pivotal trial required for regulatory approval. Participants use the device to control cursors and digital interfaces using thought, enabling independent communication and computer interaction. Neuralink’s long-term plan includes linking brain implants to spinal interfaces to restore motor control, with early animal studies demonstrating movement reactivation through direct cortical–spinal signaling.
The company is also developing Blindsight, a visual prosthesis that targets the visual cortex to restore vision in people who are fully blind. The system aims to stimulate the cortex directly to generate visual patterns, with initial versions expected to deliver low-resolution imagery and later iterations improving through denser electrodes and optimized stimulation methods. Both Link and Blindsight are part of Neuralink’s broader roadmap to restore communication, mobility, and sensory perception for people with paralysis, blindness, or neurodegenerative diseases. Over time, as safety and reliability improve, the same platform could be adapted for elective enhancement, expanding from medical restoration to applications that extend perception, cognition, and direct digital interaction.
Implant
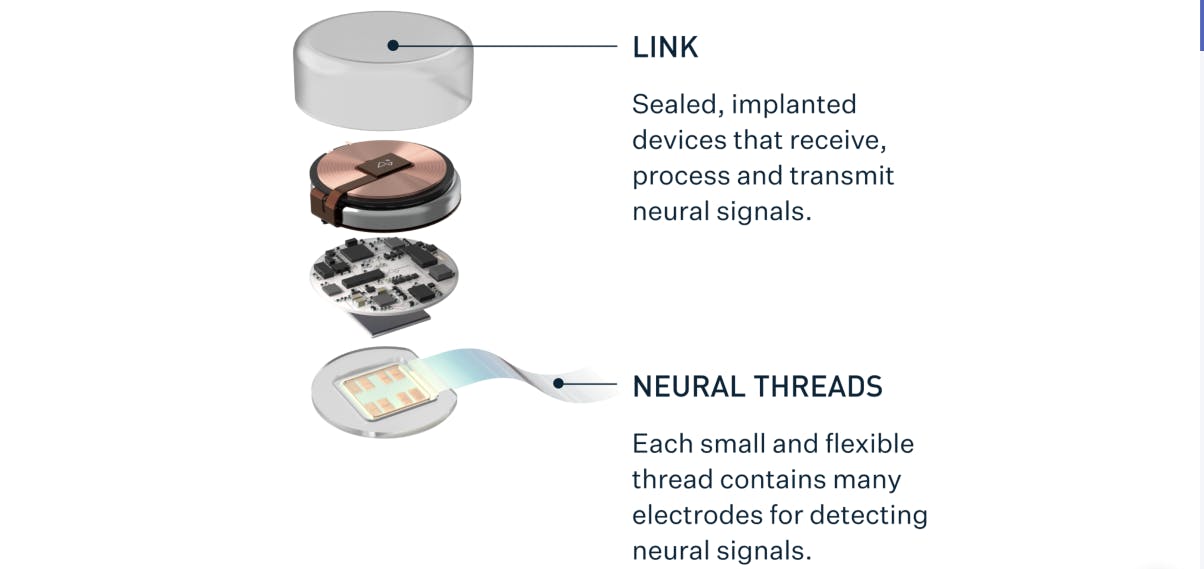
Source: Neuralink
The N1 Link implant is a fully implantable brain–computer interface that records and transmits neural activity from the motor cortex, the region of the brain responsible for voluntary movement. It works in conjunction with a surgical robot that performs implantation and an external software system that decodes recorded signals. During surgery, a circular portion of the skull is replaced with the device, which is about the size of a US quarter and nine millimeters thick. Once secured with cranial screws, it sits flush with the skull and appears as a small bump under the skin.
The device collects electrical activity from neurons through 64 polymer threads, each carrying 16 electrodes for a total of 1,024 recording and stimulation sites. The threads are extremely thin: 16–84 microns wide and under five microns thick. The threads are inserted 3–5 millimeters into the cortex using a micron-precision robotic system that avoids blood vessels to minimize tissue damage. Electrodes are spaced roughly 200 microns apart along each thread and made from alternating layers of titanium, platinum, and gold within a polyimide insulating layer, a heat-resistant plastic that maintains flexibility and electrical stability. This multilayer design provides stable contact with brain tissue while avoiding the immune response associated with rigid external connectors.
A custom integrated circuit (ASIC) inside the implant amplifies and digitizes neural signals at roughly 20 kilohertz with 10-bit precision, generating about 200 megabits per second of raw data. To reduce heat and bandwidth requirements, the ASIC performs on-chip “spike detection,” identifying moments when neurons fire and transmitting only those events. The processed signals are sent wirelessly via Bluetooth to an external computer or phone running Neuralink’s decoding software, which translates them into user actions such as moving a cursor or selecting items on a screen.
Power is supplied by a rechargeable lithium-ion battery charged inductively through the scalp. The charging system uses ferrite shielding to prevent magnetic heating, keeping surrounding tissue within two degrees Celsius of normal body temperature. The outer shell is made of polychlorotrifluoroethylene (PCTFE), a lightweight plastic similar to Teflon that allows wireless signals and power to pass through without metal casings or glass windows.
Durability testing involves accelerated aging in reactive saline at elevated temperatures to simulate years of exposure in the brain’s environment. As of August 2024, testing indicated that the implant could maintain structural integrity and performance for the equivalent of roughly ten years, though ensuring a long-term airtight seal between electronics and tissue remains a key engineering challenge. Future versions aim to increase recording capacity from 1,024 to as many as 16K channels through advances in circuit miniaturization and photolithography. Neuralink is also exploring insertion techniques that allow the robot to pass threads through the dura mater (the brain’s tough outer protective membrane) without removing it. This could reduce scarring and make future upgrades or removals simpler. Ongoing work focuses on improving signal quality, material longevity, and upgradeability to build a reliable, high-fidelity connection between neural activity and digital systems.
Surgical Placement
The surgical placement of Neuralink’s implant uses the R1 robot, a system built to insert flexible electrode threads into the brain with micron-level precision (a micron is one-thousandth of a millimeter). These threads are much thinner and softer than human hair, so they cannot be handled manually. The robot’s role is to insert each thread into the cortex at a specific depth while avoiding the tiny blood vessels that cover it.
Before surgery, participants undergo functional magnetic resonance imaging (fMRI) to locate the region of the motor cortex responsible for hand and arm movement. Even people with paralysis activate this area when they imagine moving, allowing clinicians to map it accurately. During surgery, the neurosurgeon makes a small scalp incision and removes a circular section of skull. The dura mater is then opened to expose the cortical surface. Computer vision software identifies safe insertion sites by detecting and avoiding visible blood vessels. The surgeon uses a graphical interface to outline target regions, and the robot automatically calculates the exact insertion coordinates to avoid vascular structures. The R1 robot is positioned over the site to perform the insertions. Once the threads are in place, the neurosurgeon secures the implant, replaces the skin, and closes the incision.
The R1 robot rests on a granite base weighing roughly one ton to minimize vibration. Above this base, a multi-axis arm holds optical and mechanical systems that guide the insertion process. The robot uses a light source emitting at 405 nanometers (a violet wavelength) to make the polyimide loops at the end of each thread fluoresce, helping its vision system locate them precisely. Each thread ends in a tiny loop, which the robot’s needle, with a tip only 10–12 microns wide, hooks onto for placement. The needle’s tip includes a small notch, called a “shark tooth,” that grips the loop during insertion and releases it as the needle retracts, leaving the thread embedded in brain tissue about 3–4 millimeters deep.
Although the process is largely automated, it is not fully autonomous. The robot follows a pre-programmed surgical plan, while the neurosurgeon supervises and can intervene if necessary. To prepare for operations, Neuralink engineers rehearse using synthetic models in a mock operating room. These include 3D-printed skulls based on medical scans and a hydrogel that mimics the texture of brain tissue and contains artificial blood vessels.
Recovery after implantation is typically straightforward. In the first few months, the brain forms a thin layer of scar tissue, or neo-membrane, around the insertion sites. During this early phase, the tissue remains soft enough for the threads to be removed if needed. As the scar tissue matures, the threads become anchored and stable, allowing the implant to function long-term. Removal then involves cutting the threads in place, minimizing cortical disturbance, and lifting out the implant, which can either be replaced or the opening sealed with a polymer cap.
As of August 2024, long-term studies in non-human primates indicated that leaving the threads in place is safe. Once anchored, they remain fixed and biologically inert, showing no migration or adverse effects. Neuralink has already replaced older implants in animal models without complications, supporting the feasibility of future hardware upgrades without re-entering brain tissue.
Data Collection and Processing
Neuralink’s data collection process begins at the electrodes, which measure tiny voltage changes produced when nearby neurons fire. Each electrode records activity from a few neurons within about one-tenth of a millimeter. When a neuron generates an action potential, a brief electrical pulse lasting around one thousandth of a second, the implant detects it by sampling the voltage on each of its 1,024 electrodes about 20K times per second. This high sampling rate provides a detailed picture of local brain activity and allows the system to determine when and where neurons are active.
The raw signals are processed on the implant by a custom algorithm called the Buffer Online Spike Sorter (BOSS), which identifies the characteristic waveform of each neuron’s spike amid background noise. Instead of transmitting the full data stream, which would exceed the implant’s thermal and power limits, the system sends only compact summaries that indicate when and where spikes occur. This reduces data volume while retaining the information needed to decode movement intentions.
Processing signals directly on the implant minimizes both energy use and delay. Neural activity can be detected and classified in under a microsecond, with most remaining delay coming from the Bluetooth link that transmits data to an external device. Bluetooth was chosen for compatibility with standard computers and phones, but future versions are expected to use faster, lower-latency communication methods.
To improve accuracy and durability, Neuralink is developing ways to analyze population-level signals, which reflect the combined activity of groups of neurons rather than individual spikes. This broader view provides useful context about overall brain activity, especially when single-neuron recordings weaken or fluctuate. This approach became essential after the company’s first human trial revealed that roughly 85% of the implant’s ultrathin threads had retracted slightly from the surface of the motor cortex about a month after surgery. The movement reduced the number of electrodes in close contact with neurons, weakening signal quality and cursor control. To compensate, Neuralink issued a software update that shifted the implant’s processing from detecting discrete spikes to measuring spike band power, the average activity of many nearby neurons within a frequency range. This provided a more stable signal and allowed the decoding model to be retrained, restoring and eventually improving performance.
User Experience
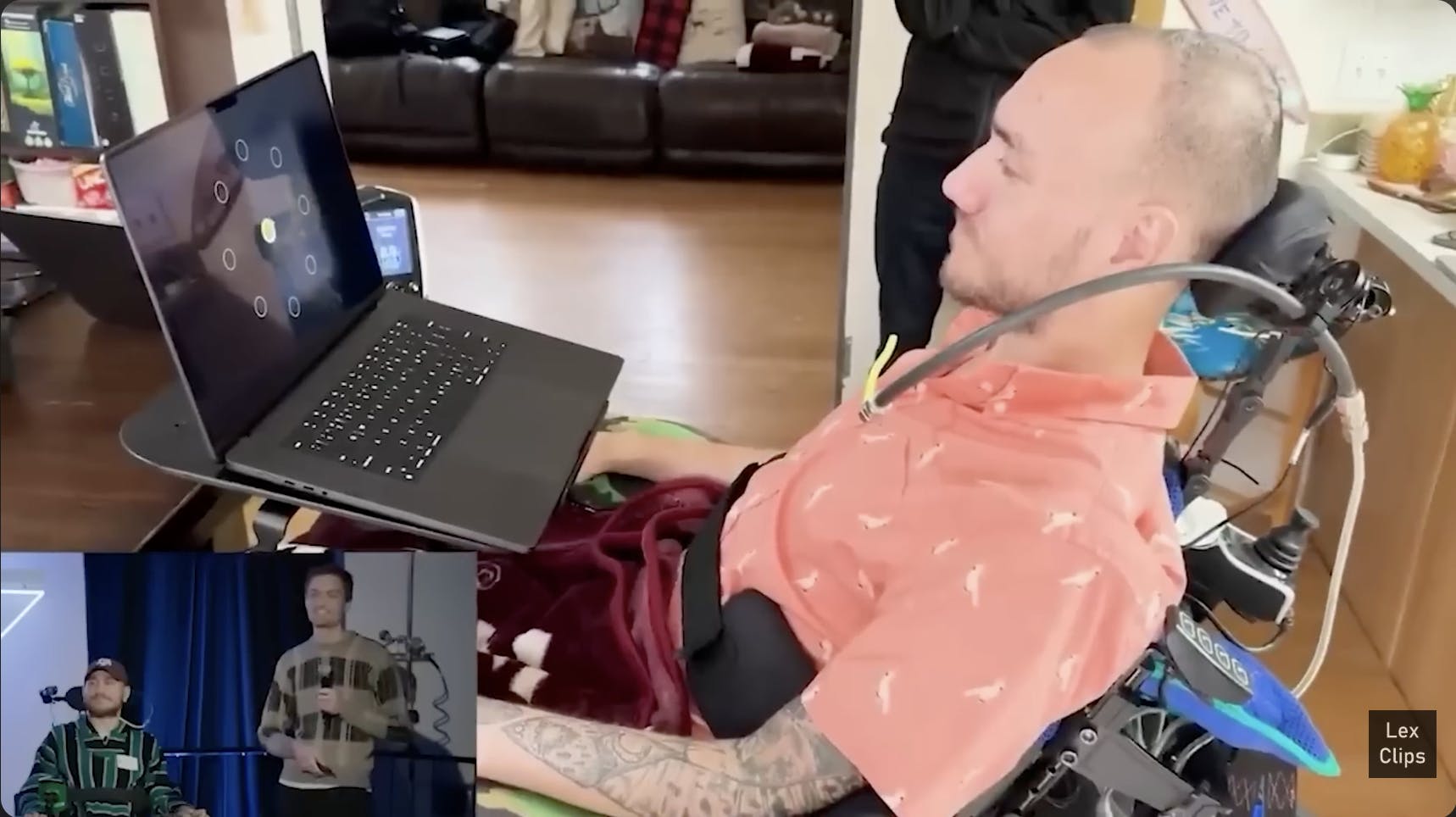
Source: YouTube
R1 App: Participants interact primarily through the Neuralink app, which receives brain activity data wirelessly from the implant and translates it into standard computer inputs such as cursor movements or keystrokes. The app also manages charging, low-power operation, and daily calibration routines. Participants can complete calibration and “body mapping” sessions independently, with results uploaded for clinical monitoring. The app can also deliver updates wirelessly. For example, after thread retraction reduced signal quality in the first human participant, the company released a firmware update that added spike band power decoding.
Calibration: Calibration teaches the software how to interpret neural activity as directional movement. It has two stages: open loop and closed loop. In open loop mode, the cursor follows preset movements on-screen while the participant imagines performing them. This pairing of known movement with corresponding brain activity provides labeled data for the decoder to learn how motor cortex patterns correspond to direction. Open-loop training also allows engineers to verify data quality before user feedback begins.
Once the system has enough data, it switches to closed-loop mode, where participants gain partial control of the cursor. Visual feedback allows both the model and user to adapt together. The algorithm refines its predictions while the participant learns which mental cues yield the best control. This co-adaptation turns the interface into a collaborative feedback system. Early in the clinical trial, high-quality calibration required 40 to 45 minutes. Through improvements to the model and user interface, the goal has been to reduce that time to under seven minutes while maintaining accuracy.
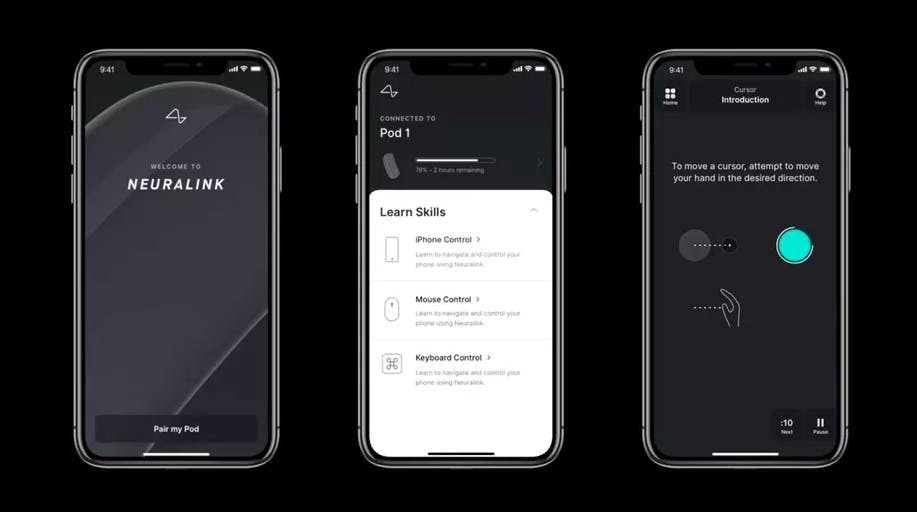
Source: Ness Labs
Body Mapping: Before calibration, the app performs body mapping to identify which parts of the motor cortex correspond to specific imagined movements. The implant records activity while the participant views animations of hand or finger motion and imagines doing the same. This process determines which electrodes carry the strongest control signals and helps track changes over time. The body mapping process begins shortly after surgery.
Cursor Control and Interface: Once calibrated, participants can operate standard computers using cursor movement, clicks, and scrolling. A custom-built “dwell cursor” registers a click when held over a target, eliminating the need for discrete click commands and improving reliability during signal fluctuations. Later updates added multiple click types and hover-based selection for smoother interaction.
Neuralink also introduced Quick Scroll, a feature that automatically detects on-screen scroll bars and converts directional neural inputs into smooth, momentum-based scrolling. This reduces unwanted motion and allows comfortable long-duration use, even with minor decoding errors. Because the system relies only on brain signals, it functions in varied conditions, such as lying down, seated, or during involuntary movements without external positioning.
Latency: As of August 2024, the system operated with about 22 milliseconds of delay from brain signal to cursor motion, faster than the roughly 75 milliseconds it takes a hand to move a mouse. The main bottleneck is Bluetooth transmission, which Neuralink plans to replace with faster protocols as performance improves.
Webgrid Benchmark: Webgrid, a target-selection game, serves as both a training and benchmarking tool. It measures information transfer using bits per second (BPS), quantifying how quickly and accurately users communicate through the interface. Previous BCIs achieved 4 to 5 BPS, while the first Neuralink participant reached 8.5 to 9.5 BPS. The test also produces large, labeled datasets that help the team refine calibration methods, evaluate latency, and improve algorithms.
Future Improvements
Future Applications: Neuralink plans to extend its work beyond motor control to speech prosthetics by decoding brain activity from regions responsible for vocal articulation. Building on academic research by Sergey Stavisky, Jaimie Henderson, and the late Krishna Shenoy, the company aims to study higher-order areas such as Broca’s and Wernicke’s.
Hardware and Surgical Improvements: Upcoming implants will be designed for greater durability, scalability, and upgradeability. The all-in-one implant design will likely shift to a modular design, with threads implanted beneath the dura and a detachable module above it containing power, processing, and communication components. This structure would allow component upgrades without re-entering brain tissue.
Neuralink is also developing techniques to insert threads through the dura rather than opening it, which could reduce scar formation and improve long-term safety. The approach depends on new needle materials and imaging systems capable of navigating blood vessels through the opaque membrane. Recording capacity is expected to increase to 16K channels. Achieving this requires finer microfabrication, lower-power circuits, and more efficient data handling, providing richer neural data for higher-fidelity control across multiple devices.
Neural Decoding: Neuralink continuously refines its decoding algorithms through user feedback. Each participant generates extensive neural data, enabling frequent model updates and rapid iteration. Users initially control cursors by imagining movement but often progress to direct neural control, focusing on the cursor itself. This adaptation informs future interface designs aimed at faster, more intuitive learning. To support scalability, the company is developing decoders that generalize across users. Standardized calibration tasks and shared behavioral vocabularies could allow models to be quickly personalized, reducing onboarding time and enabling broader clinical deployment.
Market
Customer
As of November 2025, Neuralink did not have commercial customers. As of November 2025, the company was still conducting its first human clinical trial under the FDA’s early feasibility study framework and was awaiting approval for general use. Elon Musk stated in April 2024 that he expected the implant to receive clearance within one to two years. Current implants are used only for research to evaluate safety, improve decoding software, and refine the surgical procedure.
Once FDA approval is granted, Neuralink’s first commercial users are expected to be people with paralysis or severe motor impairments, including those with spinal cord injuries and ALS. The initial application focuses on restoring what the company calls “digital freedom,” meaning the ability to control computers, smartphones, and other connected devices using only brain activity. Musk has said the aim is for someone with paralysis to operate a smartphone as quickly as, or faster than, a person typing with their hands.
Participants in the human clinical trial are individuals with severe paralysis, such as quadriplegia. Interested candidates can apply through Neuralink’s online patient registry by submitting their medical records. The company reviews applications based on inclusion and exclusion criteria to determine medical eligibility. Qualified applicants then participate in a prescreening interview and an in-home “BCI audit,” where Neuralink staff assess their living environment and the assistive technologies they already use. This step ensures the system can be used independently and safely in a home setting.
The first participant, Noland Arbaugh, works closely with Neuralink engineers to test new decoding models and user interface updates. Participants like him are considered active collaborators who provide feedback that directly shapes product design and performance improvements.
Neuralink’s long-term roadmap includes several medical and non-medical uses. The company’s vision program targets sight restoration for people who are blind through stimulation of the visual cortex. It is also exploring speech prosthetics that could decode neural signals related to vocal articulation to help people who cannot speak. Future versions may connect brain implants to devices placed in the spinal cord or peripheral nerves to restore motor and sensory function, potentially allowing individuals with paralysis to regain movement. Over time, Neuralink envisions expanding from therapeutic use to elective enhancement, offering applications that improve communication, memory, or cognitive performance.
Market Size
The global BCI market was valued at $2 billion in 2023 and, as of 2024, was expected to grow at a CAGR of 17.8% to $6.2 billion by 2030. Market growth will likely be driven by improvements in technology, along with the increasing prevalence of neurodegenerative conditions like Alzheimer's, Parkinson's, and epilepsy, and the improvement of BCI technology and its expanding applications.
Medical BCIs are anticipated to play a crucial role in addressing neurodegenerative conditions in the near future. According to the World Health Organization (WHO), the number of people living with dementia in 2017 was expected to more than triple from 50 million to 152 million by 2050, with 10 million additional cases annually. 2019 WHO estimates find that dementia costs global economies $1.3 trillion, with approximately half of the costs attributable to care provided by informal care providers (e.g., family members and close friends), who provide on average 5 hours of care and supervision per day. In addition, the WHO estimated in 2013 that between 250K and 500K people suffer a spinal cord injury (SCI) every year. An updated 2021 WHO estimate finds that approximately 15.4 million people worldwide live with SCI. Approximately 1.7% of the US population was dealing with paralysis as of 2016.
The impact of paralysis on a person's ability to effectively use computers or smartphones is significant. This limitation underscores the potential of BCIs, as they could empower individuals with paralysis to interact with technology more seamlessly. Projections indicate that affluent nations could see approximately 50 million potential beneficiaries of BCIs for medical reasons by the year 2025. This suggests a growing demand for BCI technology to enhance the quality of life for individuals with various medical conditions, ranging from neurodegenerative disorders to paralysis.
Competition
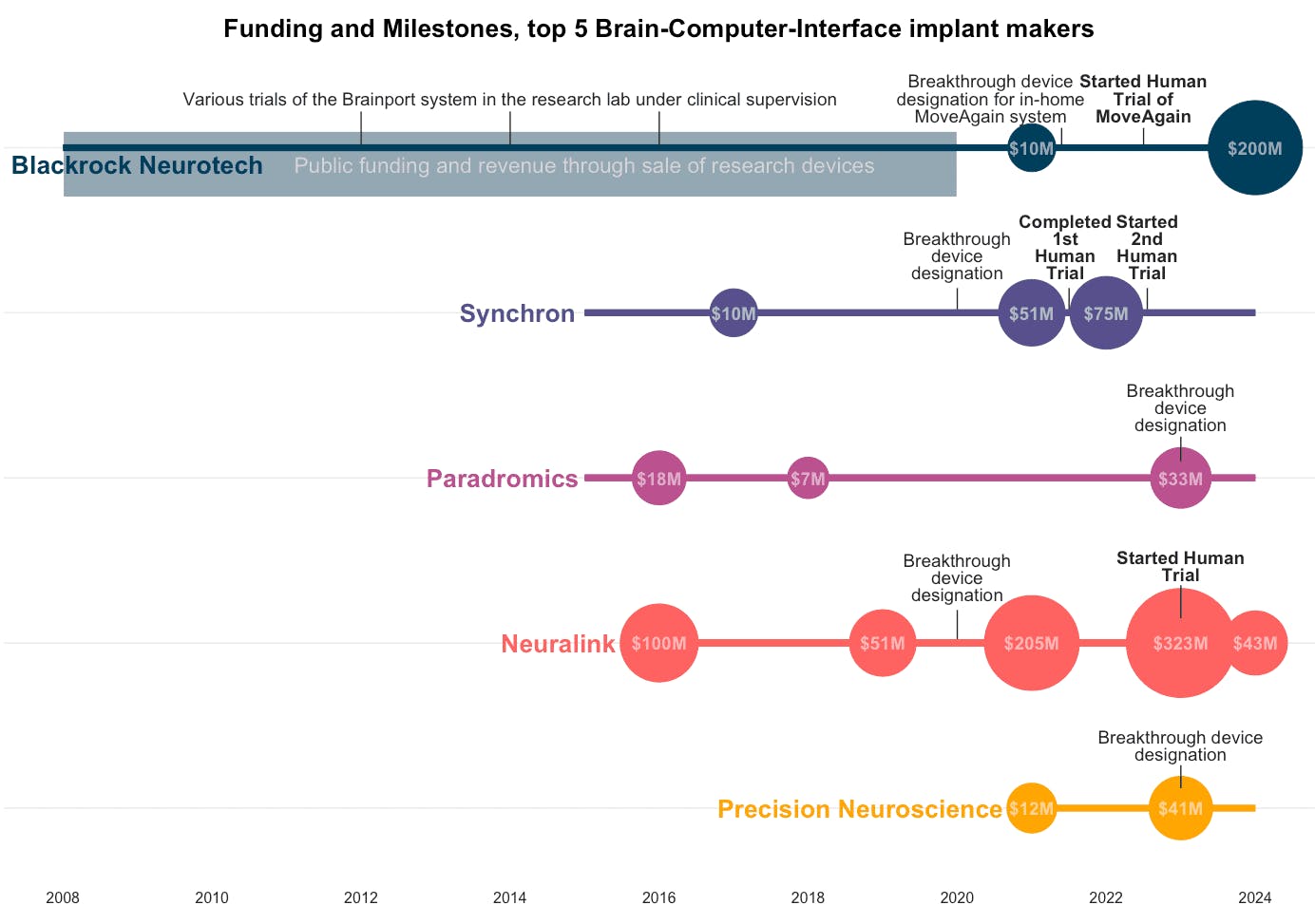
Source: From The Interface
Invasive BCI:
Science Corporation: Founded in 2021 by Neuralink co-founder and former President Max Hodak, Science Corporation, also colloquially known as Science Corp, is developing a visual prosthesis that directly competes with Neuralink’s Blindsight product. In November 2022, the company announced its flagship product, the Science Eye, which makes optic nerve cells light-sensitive through a thin, microLED display implanted over the retina. This approach allows it to bypass damaged photoreceptors to restore vision. As of October 2024, the company had completed a clinical trial for its PRIMA implant in patients with macular degeneration. The trial, called PRIMAvera, involved 38 patients and demonstrated clinically significant improvements in vision, restoring the ability to read letters and recognize faces.
In March 2025, Science Corporation raised $104 million in Series C funding, and as of November 2025, the company had raised $177.3 million in total funding. While both the Science Eye and Neuralink's Blindsight aim to restore vision, they are taking different approaches. Science Corp's technology interfaces with the optic nerve, whereas Neuralink's Blindsight bypasses the eye entirely to stimulate the visual cortex, a more ambitious strategy that could address a wider range of blindness.
Precision Neuroscience: Cofounded in 2021 by Neuralink co-founder Benjamin Rapoport, Precision Neuroscience is taking a safety-first, minimally invasive approach to BCI. Rapoport, who exited Neuralink in 2018 due to safety concerns over its penetrating electrodes, designed Precision Neuroscience’s technology to avoid direct brain tissue damage. The company’s flagship product, the Layer 7 Cortical Interface, is a thin-film electrode array that conforms to the brain's surface rather than penetrating it. This method aims to be safely reversible and less risky than more invasive implants.
This focus on a less invasive solution has enabled the company to move quickly. In May 2025, Precision Neuroscience received FDA approval for the short-term use of its device in mapping brain activity, a key step toward its development of long-term implants. In December 2024, Precision Neuroscience raised $117 million in Series C funding, and as of November 2025, the company had raised a total of $170 million from investors, including Forepoint Capital and ARCH Venture Partners.
Synchron: Founded in 2012, Synchron is a leading competitor pioneering a less invasive approach to BCI. Instead of open-brain surgery, the company has developed the Stentrode, an endovascular device that is delivered to the brain’s motor cortex via the jugular vein. This method avoids a craniectomy, enabling Synchron to progress rapidly through regulatory approval. The company received an FDA Breakthrough Device Designation in 2020 and an Investigational Device Exemption in 2021 to conduct permanent implant trials.
As of August 2025, Synchron had implanted its device in 10 people and secured a partnership with Apple. This partnership creates the first native BCI integration with Apple's ecosystem, enabling users to control iPhones, iPads, and the Vision Pro directly with their thoughts. Earlier, in July 2024, the company integrated OpenAI’s ChatGPT model into Synchron’s new chat feature for improved patient communication. As of November 2025, the company had raised $330 million in total funding. In December 2022, it raised a $75 million Series C led by ARCH Venture Partners. Other notable investors include Bill Gates, Jeff Bezos, Khosla Ventures, and the US Department of Defense.
Paradromics: Founded in 2015, Paradromics is developing a high-bandwidth BCI focused on delivering data-driven BCI technologies for brain health. Its first application is aimed at restoring communication for individuals with severe paralysis. The company's flagship product, the Connexus Direct Data Interface, is a fully implanted device that completed its first-in-human clinical trial in June 2025, focusing on patients who have lost speech due to ALS.
Paradromics' key differentiator is its focus on achieving higher data rates. While Neuralink's device has enabled cursor control at a peak performance of 10 bits per second (bps), Paradromics' BCI has demonstrated a data rate of 200+ bps. This 13-fold increase in data throughput is intended to support more sophisticated applications, such as real-time speech decoding. In February 2025, Paradromics raised a round of funding led by the NEOM Investment Fund, bringing its total capital raised to $108.4 million as of November 2025.
Blackrock Neurotech: Founded in 2008 and based in Salt Lake City, Blackrock Neurotech claims to be the first company to have its BCI clinical solutions in use. Blackrock’s core technology, the Utah Array, was first implanted in a human in 2004 and has since been used in over 32 patients worldwide as of 2022. This extensive history of human use provides the company with performance data that newer competitors lack.
While the Utah Array is a wired system, Blackrock is developing a next-generation wireless device called the Neuralace to compete with modern, fully implantable BCIs. The company, backed by Peter Thiel, received a $200 million investment from Tether Evo in April 2024. This deal gave Tether a majority stake in the company and aims to fund the commercial rollout of Blackrock's next-gen BCI technology.
Merge Labs: In August 2025, OpenAI CEO Sam Altman and Worldcoin CEO Alex Blania were in the process of co-founding Merge Labs, a startup focused on developing less invasive, high-bandwidth BCIs for human-AI integration. The company is reportedly aiming to raise $250 million at an $850 million valuation, with backing expected from OpenAI's venture arm as of August 2025.
Altman's involvement positions the BCI race as a key part of the future of artificial intelligence. Focusing on a less invasive approach, the company is set to challenge Neuralink's more invasive, surgical approach, opening a new chapter in the competition for human-AI symbiosis.
Beijing Xinzhida Neurotechnology: Emerging as a key player in China's national push into neurotechnology, Beijing Xinzhida Neurotechnology is supported by the Chinese-owned Zhongguancun Development Corporation, which generated over $1.24 billion in revenue in 2023. The company is developing an invasive BCI system focused on medical rehabilitation for conditions such as epilepsy and paralysis.
After a successful animal trial in 2024 demonstrating a monkey controlling a robotic arm, the company has moved quickly. In March 2025, Beijing Xinzhida announced that it had successfully implanted its device in three patients and was preparing to implant it in ten more. With plans for a larger trial involving 50 patients in 2026, the company is aggressively scaling its clinical program to surpass Western BCI developers in technological capabilities.
Non-Invasive BCI:
Kernel: Founded in 2016 by Bryan Johnson, Kernel is a leader in the non-invasive BCI space. Johnson, who previously founded Braintree and sold it to PayPal for $800 million, self-funded Kernel with $100 million to develop technology for measuring brain activity from outside the skull. The company's platform combines hardware and software to offer Neuroscience-as-a-Service, enabling researchers to conduct advanced studies without the need for large, traditional equipment, such as an MRI machine.
In 2020, Kernel raised a $53 million Series C led by General Catalyst, and in December 2023, it raised an additional $5.2 million, bringing its total funding to $158.2 million as of November 2025. Other notable investors include Khosla Ventures, Calm Ventures, and Eldridge Industries. Instead of pursuing direct neural control like Neuralink, Kernel focuses on providing data and biomarkers for research and wellness markets.
CTRL-labs: Founded in 2015, CTRL-labs is a New York-based company that developed a wristband that translates neuromuscular signals into machine-interpretable commands. CTRL-labs's flagship product was the CTRL-kit, a wireless, non-invasive electromyography (EMG) device that translated neural signals into control. The CTRL kit was designed for various applications, including gaming, productivity, and healthcare. The company was acquired by Meta in 2019 for between $500 million and $1 billion. Andrew Bosworth, Facebook’s head of AR, explained the vision for its product under Meta: “You have neurons in your spinal cord that send electrical signals to your hand muscles telling them to move in specific ways, such as to click a mouse or press a button.”
Neurable: Founded in 2015, Neurable is a Boston-based company spun out from the University of Michigan’s Direct-Brain Interface Laboratory (UM-DBI). The company is working on building an “everyday” BCI. The company has created smart headphones that have soft EEG sensors woven into the ear cushions. These headphones can detect patterns in brain activity with near lab-grade precision, which allows users to control their smartphones, computers, and other devices with their thoughts. Its devices also measure brain activity to generate simple, real-time insights. In April 2023, Epson Corporation invested an undisclosed amount in Neurable. In 2024, Neurable raised $13 million in a financing round led by Ultratech Capital Partners, bringing its total funding to $31 million as of November 2025. Other notable investors include Innospark Ventures, GoAhead Ventures, NXT Ventures, and the National Science Foundation.
Business Model
Neuralink’s business model appears to follow two phases: an intensive research and development stage followed by mass-market commercialization. As of November 2025, Neuralink managed all core aspects of its brain–computer interface development internally. It is focused on advancing its technology through clinical trials while redesigning its systems for scalability. Although current implementations rely on bespoke, high-cost components, the company is developing low-cost, automated manufacturing processes to enable broader adoption.
As of July 2025, mass-market commercialization was expected to begin in 2029, pending regulatory approval for the commercial use of Telepathy. The company plans to transition to a Business-to-Business-to-Consumer model, selling its complete surgical and implant system to hospitals and surgical centers. This commercial phase is supported by an internal scaling plan targeting 20K implant surgeries annually by 2031. Based on a projected $1 billion in revenue, this implies an average cost of $50K per procedure.
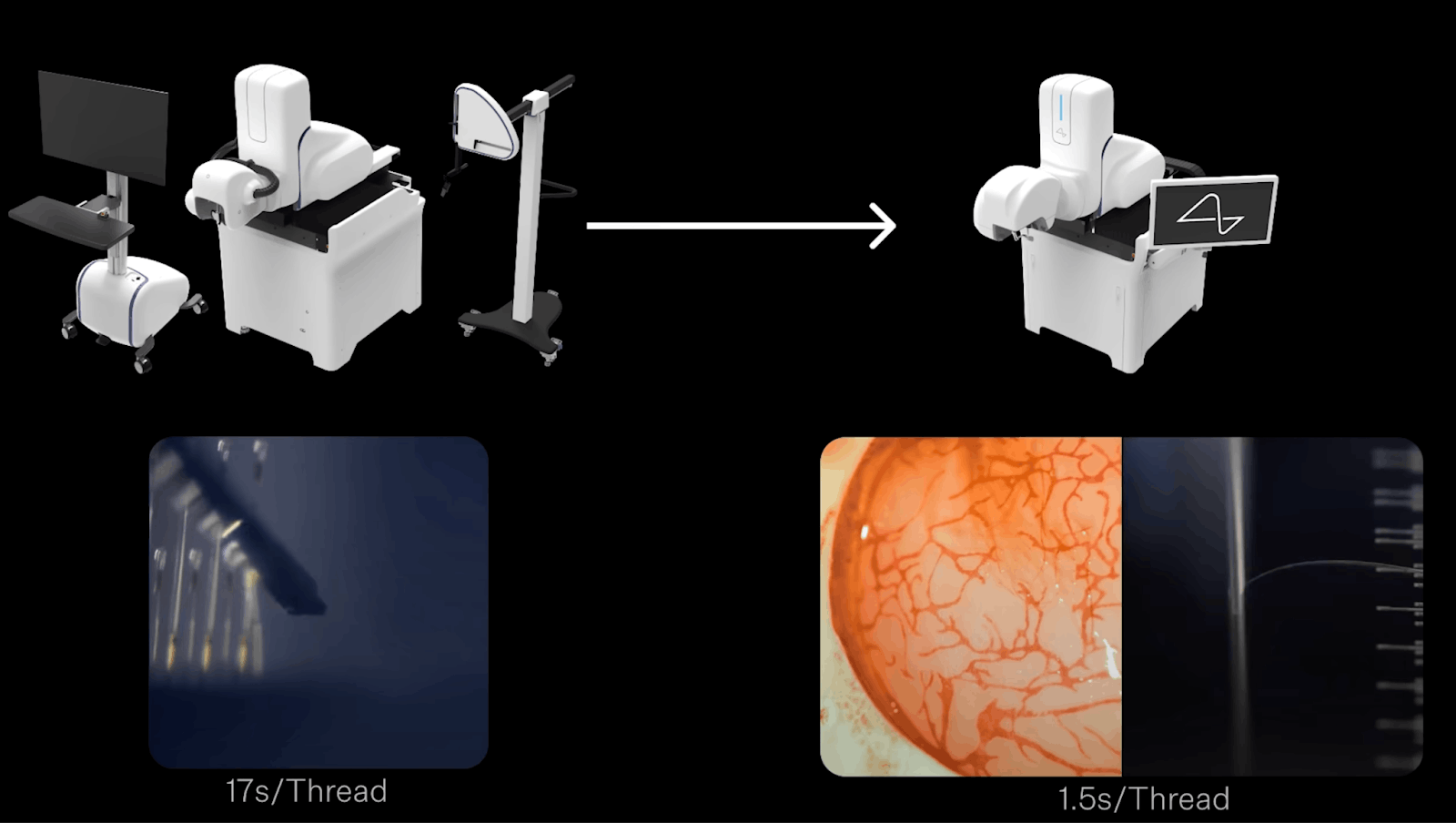
Source: Neuralink
While the initial commercial launch appears to target a $50K price point, the company’s long-term success depends on lowering component costs to enable mass adoption. This strategy is illustrated by the evolution of the R1 Surgical Robot’s needle cartridge. The first version cost $350 and required 24 hours of manual assembly, while the next-generation cartridge, designed for mass production, costs $15 and can be assembled in 30 minutes as of June 2025. Neuralink has said it aims to apply similar cost reductions across all components to reach a total procedure price comparable to Lasik, which cost $2.4K - $3.2K per eye in the US as of 2023.
Traction
Preclinical Development
Neuralink began animal testing in 2017 through a partnership with the University of California, Davis’s California National Primate Research Center. The company’s early research demonstrated its brain-computer interface (BCI) technology in pigs and macaques. In 2020, Neuralink publicly showed a live implant in a pig named Gertrude that recorded neural activity in real time, followed in 2021 by a demonstration of a monkey named Pager playing a video game using neural control. However, when Neuralink applied to begin human trials in 2022, the FDA rejected its application, citing safety and reliability concerns that required further data before approval.
FDA Approval and Human Trials
In May 2023, the FDA approved Neuralink’s first-in-human clinical trial, known as the PRIME Study (Precise Robotically Implanted Brain-Computer Interface). The study aimed to assess the safety of the N1 implant and the feasibility of restoring digital control for individuals with paralysis.
The first human implantation occurred in January 2024 in a 29-year-old quadriplegic patient, Noland Arbaugh. The device enabled him to control a cursor with his thoughts, helping him reconnect with old hobbies like gaming. The procedure marked Neuralink’s transition from animal models to human testing.
Clinical Challenges and System Recovery
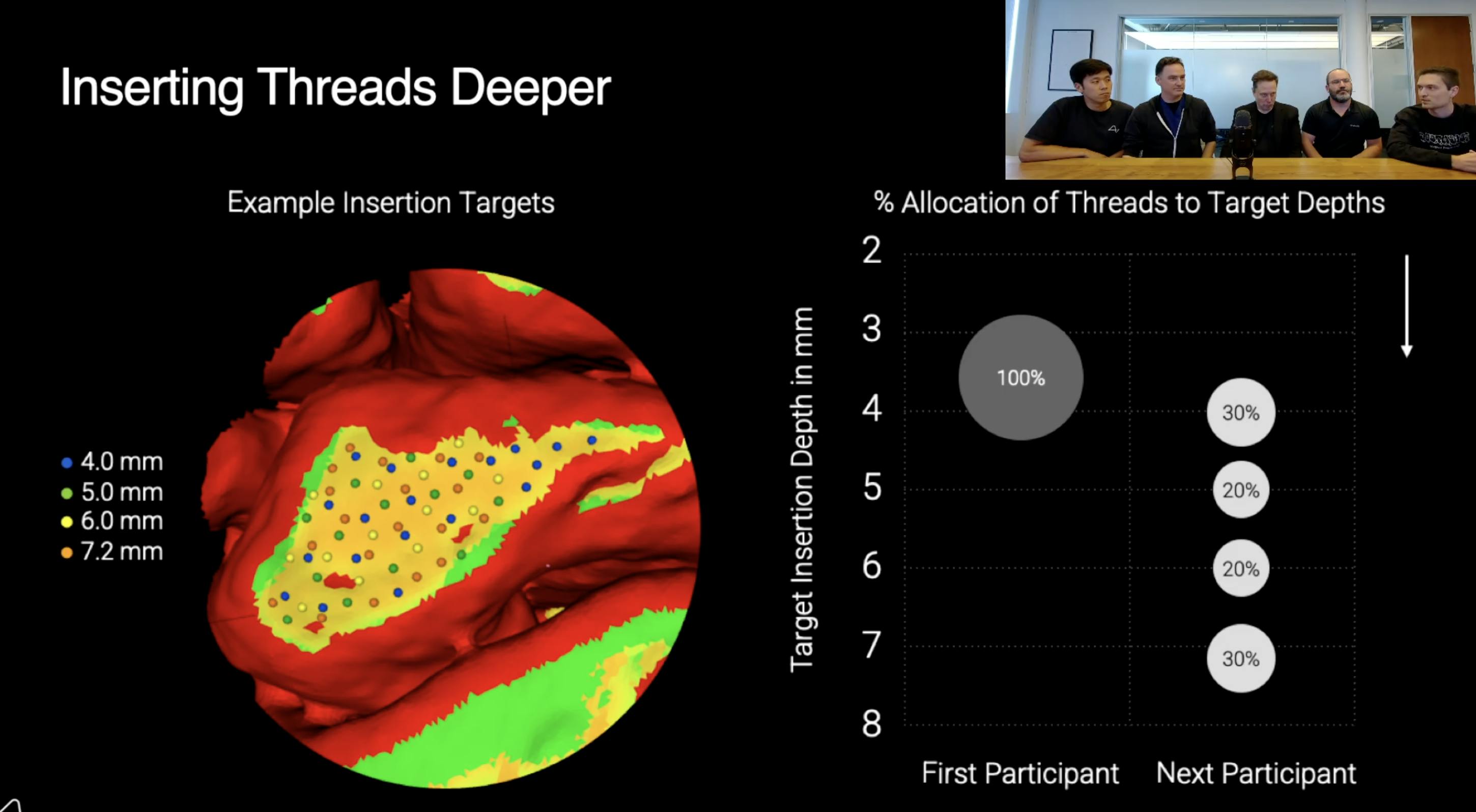
Source: X
A month after the first surgery, Neuralink reported that approximately 85% of the implanted electrodes had retracted from Arbaugh’s brain tissue, resulting in reduced signal quality. Engineers identified the issue through impedance and signal monitoring. The company mitigated the performance loss through software updates that reinterpreted neural signals and subsequently received FDA approval to implant future devices at greater depth. By mid-2024, device performance had been restored and improved through algorithmic updates. Neuralink also completed its second successful implantation and announced plans to expand the study to additional participants by the end of the year. Additionally, in September 2024, Neuralink received FDA Breakthrough Device Designation for Blindsight, with plans to conduct its first human implants within 6-12 months, as of June 2025.
Expansion and International Trials
By September 2025, Neuralink’s trial had expanded to twelve people, including individuals with spinal cord injuries and ALS. Data from the first cohort showed consistent home use of the system, averaging about 50 hours per week. The company increased its surgical cadence, performing multiple procedures within the same week, and continued to collect safety and performance data. To measure the device's real-world value, Neuralink tracks monthly hours of independent BCI usage at home. As of June 2025, data from the first five participants showed an average of approximately 50 hours of use per week, with some reaching a peak of over 100 hours.
In July 2025, Neuralink received approval from UK regulators to launch GB-PRIME, its first international clinical study. Additional trial sites were established in Canada and the United Arab Emirates. Neuralink also partnered with institutions such as the Barrow Neurological Institute to support ongoing US trials.
Product Development and Future Studies
As of November 2025, Neuralink’s development roadmap included two primary programs: Telepathy, aimed at restoring digital control for people with paralysis, and Blindsight, focused on vision restoration. Both have received FDA Breakthrough Device designations. Internal documents indicate a target for regulatory approval of Telepathy (known as Link as of November 2025) by 2029 and Blindsight by 2030. This progress also underpins an ambitious internal roadmap that targets US regulatory approval for its first commercial product, Telepathy, by 2029. According to internal documents, this is projected to be followed by the launch of Blindsight in 2030, by which time the company aims to be performing 10K surgeries annually. The long-term vision, which aligns with the PRIME study’s full completion date in 2031, is to scale to 20K implants per year, potentially generating over $1 billion in annual revenue.
Valuation
In May 2025, Neuralink raised a $650 million Series E round at an estimated $9 billion valuation. The round included participation from Founders Fund, ARK Invest, Sequoia Capital, and Thrive Capital, among others. In its announcement, Neuralink tied the new funding to several milestones achieved since its previous round, including the successful implantation of its first five patients, the launch of global clinical trials, and securing FDA Breakthrough Device Designations for new applications in vision and speech restoration.
This latest financing continues a trajectory of accelerating valuation growth. The company’s Series D in August 2023, led by Founders Fund, initially raised $280 million before being extended with an additional $43 million tranche. Since then, Neuralink’s valuation has risen in secondary markets, reaching approximately $12.7 billion as of August 2025. This increase reflects both the company’s technical progress and its stated plans to perform 20K brain implant surgeries annually by 2031.
Key Opportunities
Multi-site Implantation
Multi-site implantation provides Neuralink a potential path to extend its technology beyond single-function use. The company has tested dual implants in animals, placing devices in both hemispheres of the brain. In humans, this could involve separate implants for different functions, such as motor control and vision restoration. Standardizing the same surgical robot, materials, and core device architecture across implants could simplify development, regulatory review, and future clinical applications.
Scaling this approach, however, highlights the tradeoffs of penetrating electrode systems. Each implant covers only a small portion of the brain, so expanding functionality would require additional arrays, each displacing or damaging neurons in the process. While limited cell loss may be acceptable for severe conditions such as paralysis, broader implantation raises safety and ethical concerns. The brain’s redundancy allows for some damage, but cumulative neuron loss constrains scalability, leaving the long-term viability of multi-site invasive BCIs uncertain.
Targeting Exponential Improvements
Neuralink’s main opportunity lies in rapidly increasing the power of its BCI, a concept the company calls the “Moore's Law for neurons.” While traditional BCIs have been limited to a few hundred electrodes, Neuralink’s current N1 implant already has 1,024. This marks the beginning of an ambitious plan to increase this number to 3K by 2026, 10K by 2027, and over 25K by 2028. This increase in bandwidth is crucial for enabling more advanced applications, transitioning from basic cursor control to real-time speech decoding and beyond.
Medical Applications & Regulatory Changes
Neuralink’s near-term objective is to develop brain–computer interface (BCI) medical devices for individuals with severe physical impairments. The company’s research aligns with broader trends in using BCIs to address neurological and psychiatric conditions, including spinal cord injuries, cerebrovascular disease, sleep disorders, and mental illness. Continued progress in these areas is expected to drive overall market growth.
Source: World Economic Forum
The regulatory environment for neural devices has evolved to support clinical development and commercialization. The US Food and Drug Administration (FDA) has granted Breakthrough Device Designations to multiple neurotechnology systems, including Abbott’s deep-brain stimulation device for depression. The National Institutes of Health (NIH) funds BCI research through its BRAIN Initiative, which allocated approximately $680 million in 2023 to advance neurotechnologies.
The FDA’s updated guidance on implanted BCIs outlines safety and testing standards for devices designed for patients with paralysis or amputation. The guidance complements the agency’s Breakthrough Devices Program, which requires the FDA to respond to human-trial applications within 30 days, shortening the approval timeline for qualifying medical technologies.
The Future of Human Interaction
Neuralink may be able to create a new paradigm for human interaction by solving the "bandwidth problem." Musk argued that the main challenge in communication is that people can process information quickly, but their ability to output it is incredibly inefficient. As one Neuralink engineer explained, this limitation is similar to the early days of the internet. With a modem in the 1990s, loading a single image was slow and streaming videos was impossible. Now, with broadband, we access high-quality digital content instantly. A high-bandwidth BCI could eliminate the brain's output bottleneck, resulting in a leap in capability more profound than the shift from dial-up to broadband.
This exponential increase in bandwidth serves as the foundation for what Musk calls "consensual telepathy", the ability to communicate complex ideas directly from one brain to another without the lossy compression of language. By eliminating the physical limitations of speech and typing, Neuralink could make communication more efficient and emotionally meaningful.
Software and UX Improvements
Software and user experience improvements represent an opportunity for Neuralink to enhance the performance and reliability of its implant without additional surgery. During the first human trial, algorithmic updates alone restored and even improved signal quality after hardware complications. This demonstrated that the system’s accuracy and responsiveness can be significantly influenced by software design. Future refinements in neural decoding, such as adaptive learning models, improved noise filtering, and individualized calibration, could make control faster, more stable, and less dependent on researcher intervention.
The interface and user experience also play a critical role in translating neural intent into precise digital actions. Neuralink’s body-mapping and calibration tools show how visualization and feedback design can shape the quality of recorded signals. Continued work on intuitive interfaces, clearer feedback loops, and personalized training experiences could reduce the time needed for users to gain proficiency and expand independent use. As more participants contribute data, the company’s iterative software updates and model training are likely to drive steady performance gains and enable broader accessibility over time.
Improving Data Quality During Calibration
Improving calibration and data processing could make Neuralink’s system more accurate and practical for daily use. As of August 2024, participants completed open-loop tasks to help the implant associate specific neural patterns with cursor movements. As more data is collected from these sessions, Neuralink can refine its decoding algorithms to adjust automatically, reducing the need for researcher involvement.
Expanding the number of participants and standardizing calibration methods could help the system generalize across users, improving performance in future trials. More efficient on-device and backend processing could also allow faster updates and better handling of signal variability. These changes could strengthen reliability, shorten training time, and support broader deployment of the technology.
Key Risks
Physical Hardware Development Underpacing Software Development
Recent progress in artificial intelligence and neural decoding software has accelerated faster than advancements in implantable hardware. As of July 2024, patent and publication analyses indicate that academic research in bioelectronics has expanded rapidly over the past five years, but the rate of patent filings remains comparatively low. This gap suggests that while software-driven methods for decoding, adapting, and enhancing neural signals are maturing, the underlying hardware required for stable long-term implantation still faces unresolved scientific and engineering challenges.
Hardware limitations remain a bottleneck for commercialization. Conventional neural electrodes often suffer from poor biocompatibility and mechanical mismatch with neural tissue, which can trigger immune responses and scar formation that degrade signal quality and shorten device lifespan. Despite ongoing work on new coatings and flexible materials, the ability to create reliable, durable, and safe interfaces appears to remain limited as of July 2025. If software capabilities continue to advance more quickly than the materials and designs needed to sustain high-quality recordings, BCI companies may face constraints in translating laboratory progress into viable long-term products.
Intense Work Culture
Seven of the nine co-founders of Neuralink left the company by May 2021. This has increased competition, as notable figures like President Max Hodak and Chief of Science Benjamin Rapoport left to start their own rival BCI companies. Neuralink’s culture creates a self-imposed risk, as it has already led to the emergence of leading competitors who can now use their insider knowledge to build well-funded companies that challenge Neuralink's BCI.
Crowded Market
Though Neuralink operates with a comparative advantage in terms of media exposure, given Elon Musk’s celebrity, as well as greater funding compared to competitors, the race to successful device commercialization and broad regulatory approval remains competitive in the BCI market.
For instance, as of August 2024, competitors Blackrock (40 implants), BrainGate (15 implants), and Synchron (10 implants) had all successfully implanted more devices into patients compared with Neuralink’s total of two people. However, even as go-to-market speed remains top of mind, each company takes a different view of the right approach (non-invasive, partially invasive, and invasive) and long-term applications (solving disabilities, public commercial use, and AI-human synthesis) for BCI interfaces.
Hardware Safety & Cybersecurity
The company faces the challenge of safeguarding users’ neural signals. This introduces cybersecurity and privacy risks, ranging from the possibility of malicious actors “brainjacking” a device to worries about how neural data will be used and protected. Ensuring the safety of the implant itself while also securing digital data is a key obstacle on the way to widespread adoption.
Adoption Challenges
Another key risk to Neuralink's long-term vision is a fundamental public divide between strong support for its medical mission and deep skepticism of its cognitive enhancement goals. While 77% of Americans approve of using BCIs to treat paralysis, 83% believe implants for cognitive enhancement should be subject to stricter testing standards. This concern, fueled by worries over inequality and security, has already prompted several states to ban the mandatory use of microchip implants for employees. This creates a critical dependency: Neuralink’s ultimate goal of a consumer BCI depends on its success in the medical field. This is the most straightforward way to build the public trust needed to address the widespread ethical and safety concerns surrounding BCIs.
Invasive BCIs May Not Outperform Non-Invasive Alternatives
The assumption that penetrating brain–computer interfaces yield superior signal quality is increasingly being challenged. While invasive systems such as Neuralink’s implant can access individual neurons and capture high-bandwidth signals, this approach introduces inherent drawbacks. Penetrating electrodes cause tissue damage and scarring over time, which degrades signal fidelity and may require reimplantation. As Benjamin Rapoport, one of Neuralink’s co-founders, has noted, scaling bandwidth by adding more electrodes proportionally increases the potential for brain injury.
In contrast, recent advances in non-invasive and minimally invasive systems suggest that comparable data quality may be achievable without entering brain tissue. Electrocorticography (ECoG) devices, which rest on the surface of the cortex, and wearable EEG systems enhanced by AI decoding, ultrasound neuromodulation, and adaptive calibration have demonstrated meaningful signal improvements. For example, a 2024 Carnegie Mellon study showed that pairing a wearable EEG cap with focused ultrasound stimulation significantly improved accuracy in spelling tasks. Additionally, several commercial efforts, such as Precision Neuroscience’s surface-level implant and Synchron’s Stentrode, are pursuing clinical approval with less invasive procedures.
If non-invasive or minimally invasive systems achieve equivalent performance in decoding neural activity, invasive BCIs could lose their practical and ethical justification. The requirement for open-brain surgery, potential long-term tissue damage, and greater regulatory hurdles may limit adoption. This uncertainty poses a structural risk for companies developing penetrating implants, as emerging technologies may ultimately deliver safer, lower-cost, and more scalable solutions without compromising signal quality.
Summary
Founded in 2016 by Elon Musk, Neuralink is developing an invasive brain–computer interface (BCI) implant intended to restore neurological function in people with paralysis and related conditions. The device combines neural recording, robotic implantation, and real-time signal processing to enable communication between the brain and external devices. Neuralink began human clinical trials in 2024 and raised $650 million in Series C funding in June 2025 to expand testing and manufacturing capacity. Its near-term focus is medical, targeting movement and communication restoration, with planned extensions to vision and speech, and eventually voluntary human capability augmentation. Key challenges include competitive pressure, innovation lags, and ethical and regulatory considerations surrounding implantation and data use.